Pinnacle Edition
A powerful, multi-group, study management solution.
Exclusive Pinnacle Features
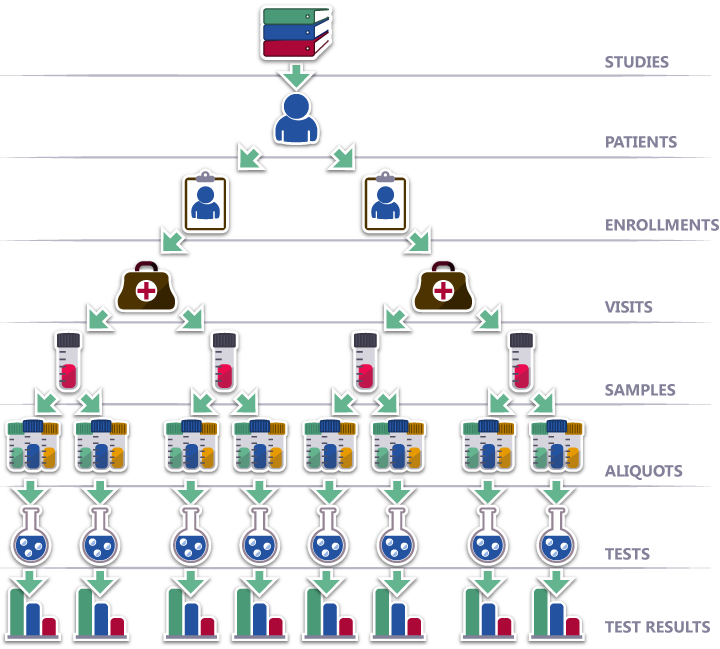
Study
Creation
Create unlimited studies with multiple arms and keep all your data in one place.
Store IRB data, set study requirements, and reversion your studies while retaining historical information.
Participant
Enrollment
Easily manage your participants at the study level.
Enroll participants in multiple studies, track consent and re-consent information, and easily adhere to regulatory requirements.
Visit
Modeling
Anticipate exactly what you need to do: template visit occurrences and pregenerate specimen kits with unique IDs.
Scan-to-receive specimens for streamlined data entry while notating specimen collection discrepancies.
Study
Health Metrics
Enjoy fully searchable kit statistics and monitor enrollment target expectations and actuals.
Get all the at-a-glance data you need with these configurable metrics.
“Our organization relies on Freezerworks to support research across multiple disciplines, each with unique biospecimen management needs.
The software’s configurability allows us to tailor fields, workflows, and access levels to fit different teams while maintaining consistency across our system. Its robust data security features and user role management ensure that sensitive information is protected, while still allowing seamless collaboration.
Additionally, Freezerworks' clinical trial management capabilities have been instrumental in keeping our biospecimen studies organized and compliant. It has become an indispensable tool for our work.”
- Leslie J Solis, MS | Assistant Director, Biorepositories Core Resource @ UC Davis Clinical Translational Science Center
A single source of truth for study management.
Pinnacle is a study management solution intentionally designed to save your team countless hours, ensure pristine data, and easily automate routine tasks.
Your team will enjoy a single source of truth for all study, patient, and specimen data so you can focus on outcomes and analysis.
Know exactly what your studies require.
Visit modeling let’s you easily anticipate what the study requires you to collect so you can pregenerate when patients should come in and what should be collected or tested during their visit.
Kit Generation
Pregenerate kits for each visit in a model and assign a unique ID to the kit to ensure it’s associated with the right participant.
Kit Labeling
Easily label at the kit, sample, and aliquot level. Scan just one label to see all the data associated with samples and aliquots.
Import Visits
Speed up your processes by importing your data as-is.